What is a Clinical Trainee (Fellow)?
-
A Clinical Fellow is a MD, MD/PhD with full or incidental patient contact
- ACGME programs (even during research-intensive years)
- And non-ACGME training programs
- Clinical Fellow appointments are set up in PeopleSoft as MF (Medical Fellow) Academic Plan, e.g. PEDSMF
- Clinical Fellows are setup with OPA, GME and other Offices
Overview of Process Steps
1. Appoint at Stanford (OPA). See online checklist:
2. https://postdocs.stanford.edu/postdoc-admins/how-appoint-postdocs
3. Set up Clinical Appointment with SHC (GME)
4. Direct Fellows to Complete Required Training: HealthStream, HIPAA, Respectful Workplace, Immunizations, and any other paperwork
5. Direct Fellows to Complete Other Training
6. Prepare Orientation Folder (see OPA toolkit online)
7. Sign-up trainees for Postdoc Benefits Orientation
8. Look up the PeopleSoft ID Number
9. Enter Paylines/Stipend/Info in GFS
Occupational Health Center Office of Environmental Health & Safety
480 Oak Road, Stanford University - (650) 725 5308
Medical surveillance appointment process for clinical trainees
1. Risk exposure assessment
- PI/Supervisor to complete online questionnaire
- https://med.stanford.edu/spectrum.html
2. Medical surveillance requirements
- Clinical trainee to search for medical records and submit documentation to OHC
- https://ehs.stanford.edu/training
3. In-clinic visit
- Schedule appointment to complete necessary testing
4. Clearance
Occupational Exposures Assessment Questionnaire
|
What to Do • PIs/Supervisors
• Identify clinical research staff’s potential exposures by completing questionnaire • Clinical research staff
• Complete all required actions outlined in OHC response |
|
PIs/Supervisors to complete questionnaire:
• Before new clinical research staff begin work |
OccupationalExposures Assessment Questionnaire
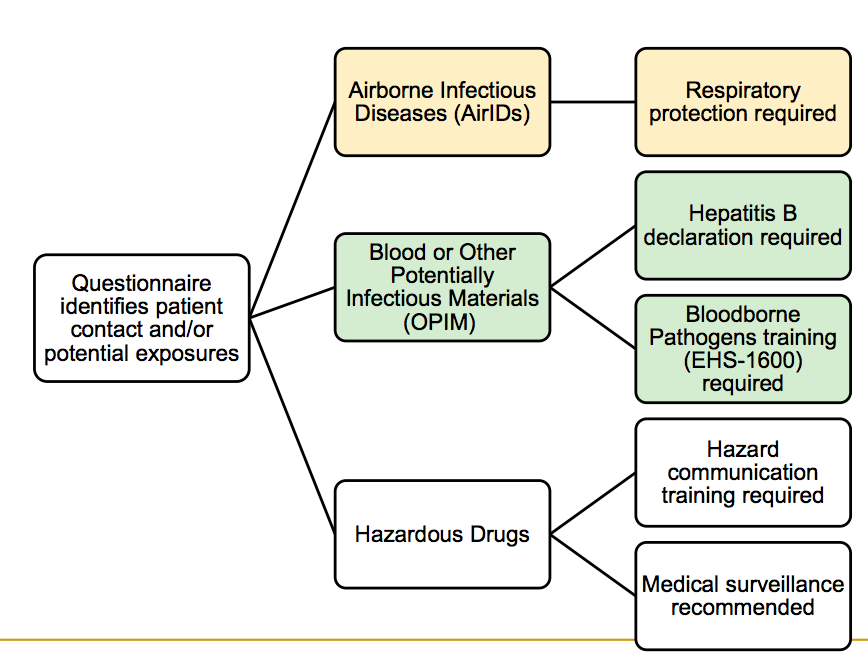
Medical surveillance requirements applicable to all clinical trainees
TUBERCULOSIS (TB) SCREENING
- TB questionnaire
-
TB testing (within last 90 days):
- Negative Quantiferon (QFT) blood test, OR
- Negative 2-step* TB skin test, OR
- If Positive test: Negative Chest X Ray is required within 1year
- TB screening is an Annual requirement
Immunizations
-
Measles-Mumps-Rubella (MMR)
- Positive Titers, OR
- Documented proof of #2 Vaccinations
-
Varicella
- Positive Titers, OR
- Documented proof of #2 Vaccinations
-
Tdap
- Documented proof of current vaccination (within 10 years)
- Signed Declaration form if there will NOT be any work with pediatric patients or LPCH
-
Annual Flu
- If vaccination is declined personnel must wear a mask when entering in patient areas during flu season
Medical surveillance requirements applicable to personnel exposed to Blood-Borne Pathogens (BBP)
HEPATITIS B SCREENING
-
Signed HepB Declaration form
- Request vaccination
- Deny vaccination
-
Proof of HepB vaccination:
- Hep B Titers
- Documented proof of 3 Hepatitis B vaccination
- Declaration form with denial and signature
Medical surveillance requirements applicable to personnel exposed to Airborne Infectious diseases
RESPIRATOR USER CLEARANCE
-
Medical Clearance
- Complete respirator user questionnaire
-
Training and fit testing
- In coordination with Industrial Hygiene
- For appointments contact EH&S
Department of Environmental Health & Safety
Reproductive & Developmental Hazard Assessment Program
- For all genders
- Assessment process
1. Confidential conference with concerned individual
2. Individual fills out Reproductive and Developmental Health Hazard Questionnaire
3. EH&S evaluates individual’s work and worksite for potential hazards (biological, chemical, radiological, ergonomic)
4. EH&S provide recommendations to individual and supervisor
- Not a requirement
- Anti-discriminatory policy
Ergonomics
Computer Workstation
1. Pay attention to changes in posture, vision
2. Complete web-based ergonomics training and workstation self-assessment (EHS-3400) to ensure proper setup
3. Take frequent micro-breaks from computer work (every 30 min for 1-2 min)
Laboratory
1. Pay attention to changes in posture
2. Take frequent micro-breaks from lab work (every 10-15 min for 1 min)
Manual Handling
1. Consult with physician/OBGYN on lifting limits
2. Use mechanical aids (e.g. carts, hand trucks, elevators)
3. Seek assistance with lifting/moving items
4. Keep loads close to the body
Contact the Ergonomics Program for additional assistance.
- Anonymous reporting, any health & safety concern: http://EHS.stanford.edu
Onboarding for Clinical Fellows
Department of Graduate Medical Education
Clinical Postdocs
- Deadline for completed packets to GME: 30 days prior to appointment start date
- Incomplete packets are not be accepted
- GME website link for Postdoc Appointments/Reappointments
Postdoc Reappointment Packets
- Approval Sheet/Email (Al Murray)
- Patient Care Form
- Updated Health Stream (Spectrum)
- Medical Board of CA License
- PDFs are preferred
- Approval Sheet/Email (Al Murray)
- Patient Care Form/Signed
- Environmental Health & Safety-Occ. Health Center
- Health Stream Clearance
- CA Medical License
- ECFMG (if applicable)
- Medical School Diploma
- CV
Visas
J-1 visas
- Completed materials sent to GME as separate PDFs
- www.ecfmg.org
Graduate Medical Education
- Reviews credentials
- Enters into MedHub
- Requests LPCH EPIC access
Concurrent Clinical Postdoc Trainee & Clinical Scholar Appointments
School of Medicine Faculty Handbook Chapter 3.2.E.3.a
- Concurrent Fellow / Clinical Scholar appointments are for exceptional circumstances to recognize the candidate's level of contribution to the teaching program in a capacity other than as a trainee or a Postdoctoral Scholar who wishes to maintain currency of clinical skills
Sequence of Events
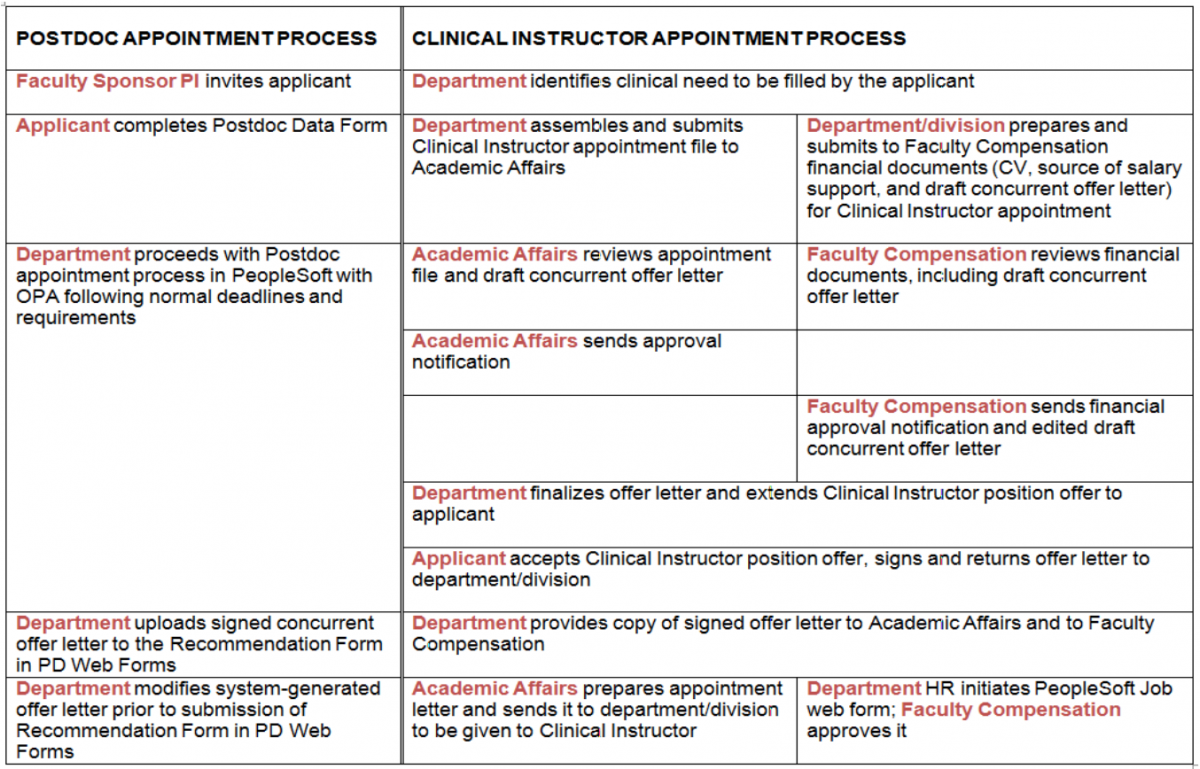
Required Documents
- Appointment file is same as for any new Clinical Scholar appointment
- Appointment forms and templates
- Compensation documents: concurrent offer letter
Concurrent Offer Letter & Appendix
- Expectations and Responsibilities
- Appointment
- Assignment and Responsibilities
- Funding and Compensation
- Benefits
- Leave
- Requirements
Important Reminders
1. Must upload joint offer letter to the Recommendation Form in PD Web Forms.
2. Prior to submission of Recommendation Form in PD Web Forms, insert sentence into offer letter stating:
“Online acceptance of this offer letter is superseded by the joint Postdoc/Clinician Educator offer letter issued by Offices of Academic Affairs and Postdoctoral Affairs and signed by you”
Special Case of Concurrent Fellow/ Clinical Scholar (Affiliated)
- To recognize the candidate's level of contribution to the teaching program in a capacity other than as a trainee
- These individuals are NOT salaried employees of Stanford University and are NOT being paid in any way by Stanford; such as receiving a stipend for their Fellowship appointment.
- OAA does not require an offer letter for Clinical Scholar (Affiliated) appointments, therefore, Faculty Compensation is not involved in these actions.
- Clinician Educator (Affiliated) letter of invitation template
- IMPORTANT REMINDER: Since there is no concurrent offer letter the postdoc admin must upload a copy of the letter of invitation sent to the Clinical Scholar (Affiliated), as well as the email notification from OAA, to the Recommendation Form in PD Web Forms.
Appointing Clinical Trainees (Fellows)
When to Appoint Clinical Trainees through OPA?
- For an “Academic Affiliation” with Stanford University.
- If funding will come from Stanford University sources (Training Grants…)
- To facilitate eligibility for the Trainees to apply for external fellowships through RMG (such as American Heart Association)
A Clinical Fellow appointment in OPA should be concurrent with a GME appointment.
A GME appointment of a Clinical Fellow does not require a concurrent appointment in OPA.
Who Is Involved in a Clinical Trainee Appointment
Stanford University staff who are designated with approval from OPA as Postdoctoral Administrators
Staff who initiate the process must be:
1. Authorized users of PeopleSoft
2. Able to review and upload documents to PeopleSoft
3. A point of contact for the Fellows regarding requirements, process steps and arrival orientation
An academic org code must exist for the division with the University
Approval roles must be established for the division
Responsibilities of Postdoc Administrator
Complete Required Training and Get Access to PeopleSoft
1. PeopleSoft “Concepts & Compliance”.
2. Postdoc Policies and Procedures
3. Postdoc Web Forms
Postdoc Policies and Procedures and Postdoc Web Forms prerequisites can be satisfied by attending the PeopleSoft Open Lab, 1st Friday of each month at MSOB 1265 Welch Road, between 8:30am and 10:30am
Your DFA or Division Manager (role 3 approver of Postdoc Web Forms) must request authority for you by submitting a HelpSU ticket to: Request Category: Student Services, Request Type: Postdoctoral Affairs.
Information about the next training session will be given in HelpSU resolutionresponse to your DFA or Division Manager.
Responsibilities of Postdoc Administrator
Collect information from the sponsoring faculty and trainee:
- Non-Stanford Email address of the clinical trainee to initiate appointment.
- Appointment start and end dates, funding amounts and sources, and any other special terms and conditions of appointment.
Be point of contact for the trainee on the process and training requirements
- Give Stanford student ID to trainee
- Monitor completion of all required training
- Submit immunization data to Occupational Health
- Follow checklist of required payroll and other setup documents
Required Paperwork to Upload in PS for OPA’s Review
1. Copy of the diploma or degree completion letter
2. External funding letters
3. Recent and complete curriculum vitae
4. Copy of CA medical license. A printout of a valid license from www.medbd.ca.gov is acceptable.
5. Signed Patient Care Form
http://postdocs.stanford.edu/admin/pdfforms/Patient_Care_Form_2012.pdf
6. Agreement for Services Outside of Fellowship– submit only if the trainee will bill for services. Billing must be outside of the fellowship program scope. http://postdocs.stanford.edu/admin/pdfforms/MD_AgreementBilling.pdf
Steps to Process an Appointment
1. PD Web Forms is the method for submission of appointment.
2. All information entered in Postdoc Data Form online by Trainee
3. Trainee uploads supporting documents (PDF preferred) to the Data Form and submits it.
4. Administrator receives Data Form, reviews it and approves if correct, or returns if incorrect/incomplete.
5. Administrator initiates the Recommendation Form after approving Data Form.
6. Administrator collects any missing and additional documents.
7. Administrator submits completed work for approval to DFA/Other Designee. APPROVAL BY DFA GENERATES NOTIFICATION TO TRAINEE TO REVIEW/ACCEPT OFFER LETTER.
8. Upon acceptance of letter by trainee, Administrator approves so that transaction is routed to OPA for final review.
9. OPA approves completed and correct submissions within 5 days. Approval email is sent to Administrator and the Trainee.
10. Administrator submits complete packet to GME in PDF format

Submissions must be complete and correct by deadline. OPA will return incomplete or incorrect transactions in Workflow.
Role 3 DFA must re-approve the appointment and Trainee must re-accept the offer letter
Funding Policy for Clinical Trainees
- ALL Clinical Fellows must receive the appropriate PGY level support, whether they are appointed at OPA or at GME.
- Clinical Fellows in a research-intensive period in a clinical training program must receive the appropriate PGY level of support; i.e., no reduction in pay is allowable because the clinical trainee is doing research.
- A combination of School and SHC/VA sources may be used towards the PGY support. All funding sources must be entered in GFS. Direct-paid SHC/VA funding must be entered as “info-only” lines.
Important Funding Guidelines
- Only funding that is paid towards support of the training program is counted as part of the PGY level. In other words, pay for moonlighting or other work at the clinics that is outside of the training program DOES NOT count towards the funding minimum required towards training.
- Include all special funding terms and conditions (e.g., clinic time, on-call expectations…) in the offer letter.
- Billing in the area of training is NOT ALLOWABLE.
Paying Clinical Trainees in GFS
- Department/Division GFS Administrator aid-year activates and enters funding information in GFS and enters FLSHP TUITION or Approves TAL for tuition fees.
- Funding information in GFS must include any Info Only Lines for support coming from GME/SHC.
- Total funding level must meet appropriate PGY scaleTotal funding can be a combination of SoM and SHC sources
- A $125 per term Registration fee is paid on their behalf.
Exceptions to Clinical Appointments
A written justification request from faculty sponsor, uploaded with the appointment documents online, is required in the following cases:
- MD was conferred more than 6 years ago
- An appointment is requested at less than 100%
- Non-standard curriculum
- Foreign Nationals (require special review and additional approvals)
Non-U.S. (foreign) MD Clinical Trainees
- Can be clinical trainees with MD credentials from another country.
- Require CA Med Board License Section 2111 exemption approved by ECFMG and processed through GME. Must obtain GME Pre-Approval first.
- The J-1 visas must be approved by ECFMG, processed through GME
- May not bill for services
NOTE: A foreign MD in the US on a Research Scholar J1 visa CANNOT switch to a Clinical Trainee with a J1 Clinical Scholar Visa. Consult with Bechtel if questions.
MDs on Visas – J-1 Visa Status
- ECFMG will allow maximum of seven years of clinical scholar program
- MDs cannot be appointed to a research appointment (without ECFMG approval) prior to a clinical appointment as this is considered a change in program
- Completion of studies and two year return home requirement:
- J-1 Research Scholar status
- J-1 Clinical Scholar status
Clinical Trainees at the VA or PAVIR
Stanford Policy Applies:
- Must be appointed by Stanford faculty member
- Same Clinical Fellow appointment process
- Supporting documents must include VA proof of support
Usually waive Postdoc Benefits; waiver form is still needed.